39 multistep reaction energy profile
5.10 Multistep reaction energy profiles.pdf - Course Hero 5.10 Multistep reaction energy profile Review: Most reactions have multiple steps The rate of reaction is determined by the slowest step In a single step reaction, you see a simple energy diagram like the one on the right: In multi-step reactions, each elementary step has its own activated complex and its own activation energy barrier. The slow step has an activation energy barrier that is ... Multistep reaction energy profiles | Kinetics | AP Chemistry | Khan ... Many chemical reactions have mechanisms that consist of multiple elementary steps. The energy profile for a multistep reaction can be used to compare the act...
autodE: Automated Calculation of Reaction Energy Profiles— Application ... Calculating reaction energy profiles to aid in mechanistic elucidation has long been the domain of the expert computational chemist. ... The general applicability of autodE is demonstrated in complex multi-step reactions, including cobalt- and rhodium-catalyzed hydroformylation and an Ireland-Claisen rearrangement.

Multistep reaction energy profile
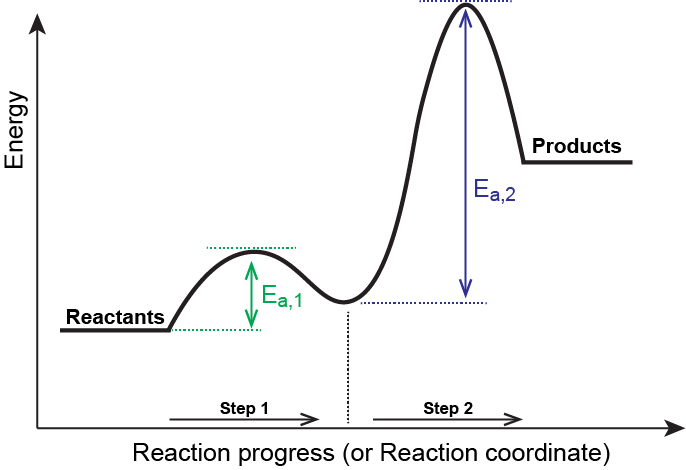
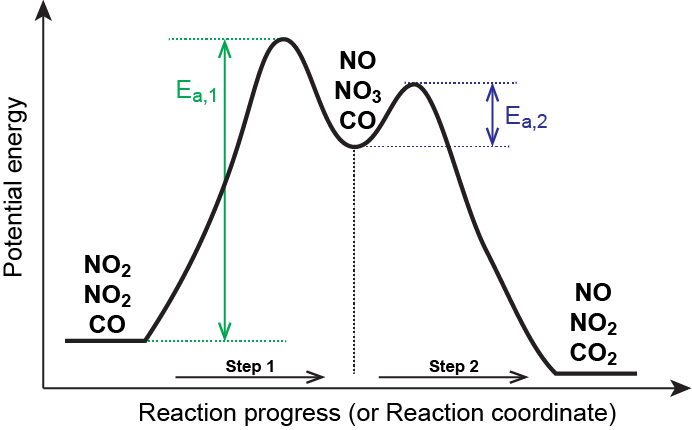
Multistep Reaction Energy Profile | StudyAPChemistry This is an exothermic reaction, as heat energy is released. We will cover this more in the next unit, which is Thermodynamics. That's basically it for the Multistep Reaction Energy Profile. If you are interested in moving onto Catalysts, which plays a big part in Kinetics, please take a look at the next guide, which is the final one for this unit. PDF Unit 5 - Kinetics - Chemistry Teaching Resources The previous diagram shows a typical reaction pathway for a multi-step reaction, in which the first stepis the slowest step(highest activation energy). The diagram below illustrates the situation where the slowest step is not the first step. AP e e a 2021 page 73 e 1.hen free ClW (g) atoms encounter O 3(g) Collision theory and the Maxwell-Boltzmann distribution - Khan Academy On an energy profile, we have the reactants over here in the left. So A, atom A is colored red, and we have molecule BC over here, So these two particles must collide for the reaction to occur, and they must collide with enough energy to overcome the activation energy barrier.
Multistep reaction energy profile. USATestprep: K-12 standards-aligned practice tests (5.6) Reaction Energy Profile (5.7) Introduction to Reaction Mechanisms (5.8) Reaction Mechanism and Rate Law (5.9) Steady-State Approximation (5.10) Multistep Reaction Energy Profile (5.11) Catalysis; Thermodynamics (6.1) Endothermic and Exothermic Processes (6.2) Energy Diagrams (6.3) Heat Transfer and Thermal Equilibrium Chapter 19 Chem 2 Flashcards | Quizlet Correctly order the steps required to derive the overall rate law for a multistep reaction that has one or more fast initial steps. 1. Express the equation of each fast step preceding the rate-determine step as an equilibrium process ... There will be three peaks in the energy profile The energy of the products will be lower than the energy of ... Lesson Explainer: Reaction Profiles | Nagwa The reaction of hydrogen gas with oxygen gas to produce water is an exothermic reaction, producing 285.8 kJ/mol of energy per mole of hydrogen gas. The chemical equation for this reaction can be written as H () + O () H O () k J m o l 2 2 2 g g l 1 2 + 2 8 5. 8 / Using this information, the following energy level diagram can be constructed. Analyzing Multi-step Reaction Energy Profiles - Study.com Analyzing Multi-step Reaction Energy Profiles High School Chemistry Skills Practice 1. Considering the graph, how many elementary steps are in the reaction mechanism? 2. On the following multi-step...
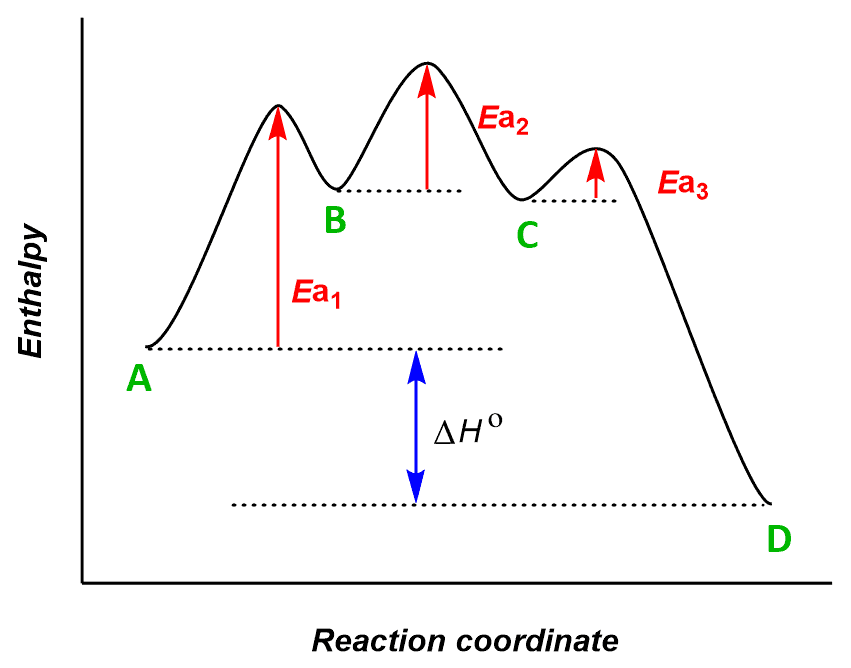
AP Chemistry Course Description / Latest Syllabus (2022) 5.6 Reaction Energy Profile. 5.7 Introduction to Reaction Mechanisms. 5.8 Reaction Mechanism and Rate Law. 5.9 Steady-State Approximation. 5.10 Multistep Reaction Energy Profile. 5.11 Catalysis. Unit 6: Thermodynamics. 6.1 Endothermic and Exothermic Processes. 6.2 Energy Diagrams. 6.3 Heat Transfer and Thermal Equilibrium. 6.4 Heat Capacity and ... Exploring Reaction Energy Profiles Using the Molecules-in-Molecules ... to study the potential energy profiles for multistep chemical reactions using the MIM methodology. In a complex multistep chemical reaction, the fragmentation scheme needs to be changed as the reacting species transition into a new reaction step, resulting in a discontinuity in the potential energy curve of the reaction. In our approach, the Multistep reaction energy profiles (video) - Khan Academy Many chemical reactions have mechanisms that consist of multiple elementary steps. The energy profile for a multistep reaction can be used to compare the activation energies of the different steps and identify the rate-determining step. The energy profile can also be used to determine the overall change in energy for the reaction. PDF Unit 5 - Kinetics of a chemical reaction on temperature can be expressed by the following equation, known as the Arrhenius equation k = Ae -E a /RT here, w A = collision frequency (can be considered a constant) E a = activation energy R = the gas constant (8.314 J K -1 mol -1 ) T = absolute temperature (in K) Taking ln
Multistep Reaction — autodE documentation - GitHub Pages *args - Set of reactions to calculate the reaction profile for calculate_reaction_profile ( units : Union [ autode.units.Unit , str ] = 'kcal mol-1' ) → None # Calculate a multistep reaction profile using the products of step 1 as the reactants of step 2 etc. Example Solved The energy profile diagram below of a multi-step - Chegg The energy profile diagram below of a multi-step reaction Examine the diagram and answer the following questions B Gibbs Energy M У А G Reaction progress I. How many steps are there in this reaction? Less Is the first step in the reaction endergonic or exergonic? autodE: Automated Calculation of Reaction Energy Profiles ... - PubMed The general applicability of autodE is demonstrated in complex multi-step reactions, including cobalt- and rhodium-catalyzed hydroformylation and an Ireland-Claisen rearrangement. Keywords: automation; reaction mechanisms; sampling; transition states. Exploring Reaction Energy Profiles Using the Molecules-in-Molecules ... The present work delineates a protocol to study the potential energy profiles for multistep chemical reactions using the MIM methodology. In a complex multistep chemical reaction, the fragmentation scheme needs to be changed as the reacting species transition into a new reaction step, resulting in a discontinuity in the potential energy curve ...
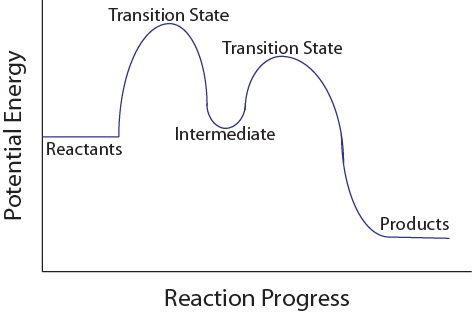
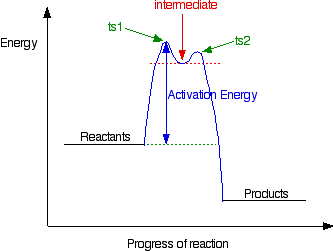
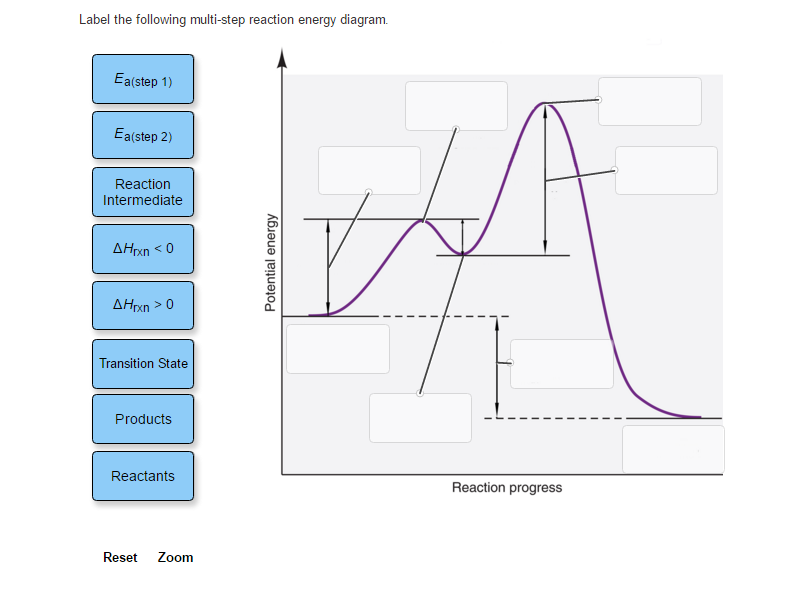
5.10+Multistep+Reaction+Profile+Diagram+Student.pdf - 5.10... NOTES: Reaction energy profiles can also be used to illustrate a multistep reaction, as long as the energetics for each step are known. In a two-step reaction, two transition states are shown. In a three-step mechanism, three transitionstates are shown.
CHEM 211 Ch.14 Flashcards | Quizlet -When particles collide, some of their kinetic energy is converted to vibrational energy. -Reaction rate is directly proportional to the number of collisions per second. Which of the following factors are affected by an increase in reactant concentration? -The number of reactant collisions -The number of reactant particles in a given volume
Multistep Reaction: Definition & Energy Profile | StudySmarter The Multistep Reaction Mechanism The formula below is an example of a net chemical equation: 2 H 2 + 2 N O → 2 H 2 O + N 2 There are three total steps in this reaction. Our reactants are that "first domino" that knocks over all the others until we get to the "final domino", our products. It makes sense that this reaction takes many steps.
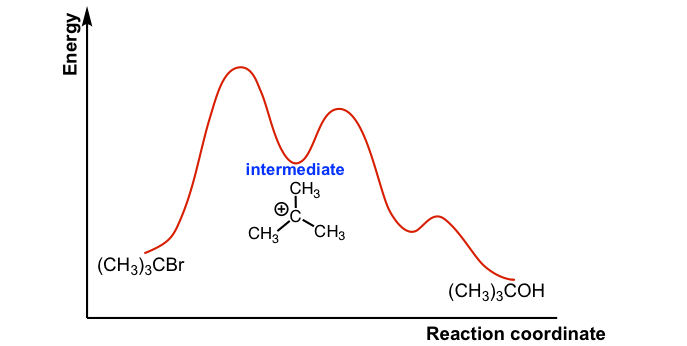
ENERGY PROFILES FOR SIMPLE REACTIONS - chemguide Energy profiles for reactions which go via a single transition state only This is much easier to talk about with a real example. The equation below shows an organic chemistry reaction in which a bromine atom is being replaced by an OH group in an organic compound. The starting compound is bromoethane, and the organic product is ethanol.
Energy Profile: Definition, Diagram, Reaction | StudySmarter The last type of energy profile we will cover is for multistep reactions. These are reactions that, like the same suggests, have several steps that proceed in order. Think of it like knocking down dominoes: As one falls, the other is knocked over. The catalyst reaction we saw before is an example of a multistep reaction.
Multistep Reactions - Softschools.com The rate of a multistep reaction depends on what species are involved before the slowest (rate-determining) step. Species can be formed, then consumed, in the reaction, but do not exist for a long period of time and do not appear in the overall reaction equation. These transient species are called intermediates.
PDF MULTISTEP REACTIONS - msu.ru MULTISTEP REACTIONS 1. INTRODUCTION ТЬе DWA as used in Chapter V and Section VI.3 describes а single-step reaction as exhibited Ьу the explicit арреагапсе of the responsible interaction only опсе in the transition matrix element [see (У.4.8) and (VI.2.26')]. Моге picturesquely, опе visualizes the incident projectile passing пеаг
A Guide to AP® Chemistry Units & Topics - UWorld College Prep 5.10 Multistep Reaction Energy Profile: Learn to depict the reaction energy profiles of multistep reactions, showing the activation energy and overall energy change of the reaction. 3. Representing Data and Phenomena: 5.11 Catalysis: Learn how catalysts affect reactions by altering activation energy and/or reaction mechanisms. 6. Argumentation
Analyzing Multi-step Reaction Energy Profiles | Chemistry | Study.com In a reaction energy profile, this is shown as everything between two low points. Intermediate: In a multi-step reaction, this is a relatively stable or low energy state and is shown by a low point...

How to Draw Multi-Steps Energy Profile Diagrams: Reactant, Product, ∆H, Activation Energy, Slow Step
5.10 - Multistep Reaction Energy Profiles - YouTube Notes graphic organizer can be found at: video reviews how a reaction ...
Collision theory and the Maxwell-Boltzmann distribution - Khan Academy On an energy profile, we have the reactants over here in the left. So A, atom A is colored red, and we have molecule BC over here, So these two particles must collide for the reaction to occur, and they must collide with enough energy to overcome the activation energy barrier.
PDF Unit 5 - Kinetics - Chemistry Teaching Resources The previous diagram shows a typical reaction pathway for a multi-step reaction, in which the first stepis the slowest step(highest activation energy). The diagram below illustrates the situation where the slowest step is not the first step. AP e e a 2021 page 73 e 1.hen free ClW (g) atoms encounter O 3(g)
Multistep Reaction Energy Profile | StudyAPChemistry This is an exothermic reaction, as heat energy is released. We will cover this more in the next unit, which is Thermodynamics. That's basically it for the Multistep Reaction Energy Profile. If you are interested in moving onto Catalysts, which plays a big part in Kinetics, please take a look at the next guide, which is the final one for this unit.
























Komentar
Posting Komentar